Ocean acidification would refer to a drop in the pH of the oceans, which is attributable to the absorption of anthropogenic carbon dioxide emissions from the atmosphere. Coral reef calcification has also slowed due to acidification of seawater. Dissolved calcium carbonate powder can exacerbate reef loss, but this is currently difficult to control. We found that calcium carbonate dissolution at five coral reef distribution sites around the world is inversely proportional to the aragonite saturation state (Ωar) covering the seawater, and that the solubility of calcium carbonate sediments is 10 times more sensitive to seawater acidification than coral calcification, so when The aragonite saturation state of seawater reaches 2.92+0.16 (estimated in 2050). The global reef sediments will transition from precipitation to net dissolution. Notably, some reefs have already experienced sediment dissolution.
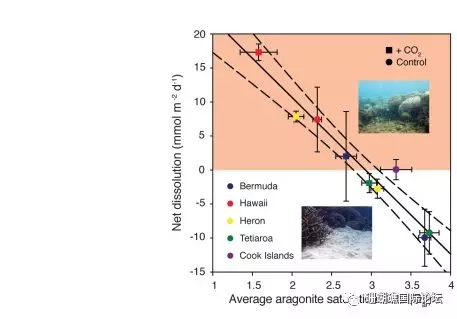
Figure 1 Relationship between the average dissolution rate of calcium carbonate permeable sediments and the saturation state of seawater aragonite (Ωar) for five coral reefs
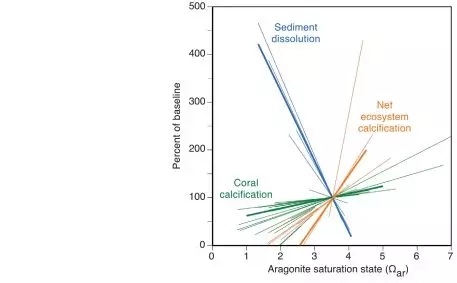
Figure 2 The relationship between the dissolution rate of permeable sediments in coral reefs, the calcification rate of coral, the total calcification rate (NEC) of the ecosystem and the saturation state of seawater aragonite (Ωar)
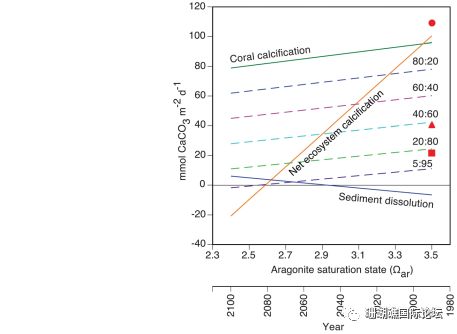
Figure 3 Correlation of coral calcification rate, sediment dissolution rate, total ecosystem calcification rate and seawater aragonite saturation (Ω) around coral reefs around the world.
Source: "Science"